Novo Nordisk's Ozempic Receives FDA Approval as a Kidney Disease Treatment
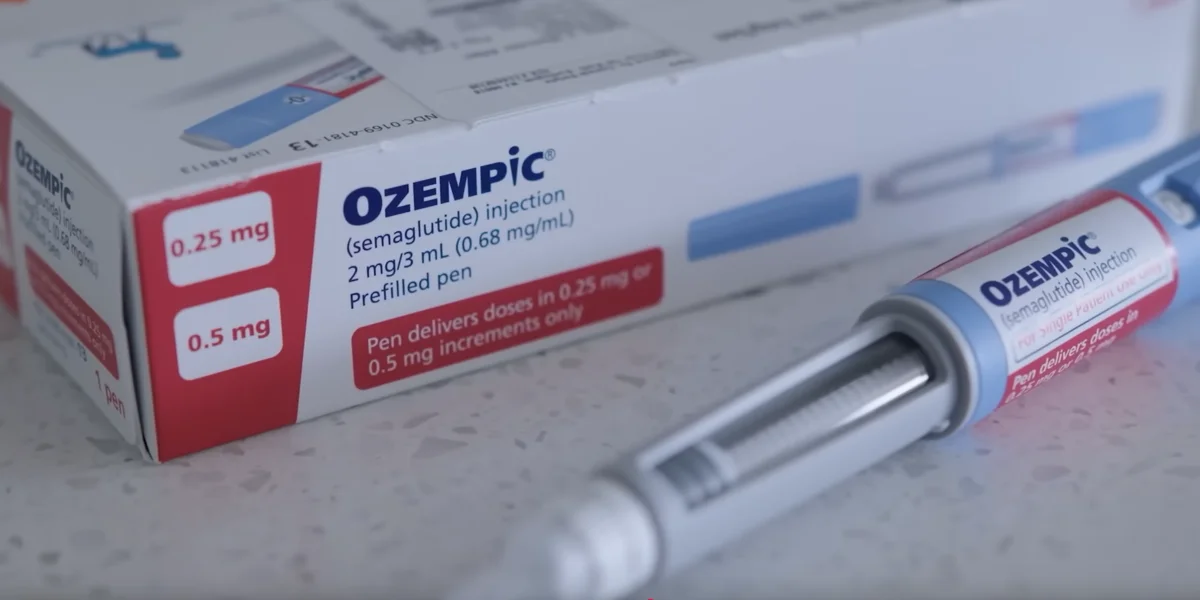
Ozempic, a blockbuster GLP-1 medicine initially licensed to treat type 2 diabetes, has now been approved by the US Food and medicine Administration to lower some risks associated with chronic kidney disease, Novo Nordisk announced Tuesday.
A Phase 3 clinical research discovered that injecting the semaglutide medicine once weekly decreased the chance of renal disease deteriorating by 24% in persons with diabetes. It also lowered the risk of kidney failure and heart disease mortality by roughly 5% in persons with both type 2 diabetes and chronic renal disease, according to a company press statement.
The ruling might change the way doctors treat patients with chronic renal disease, which causes progressive loss of kidney function and is one of the top causes of mortality in the United States. Novo Nordisk estimates that around 37 million American people suffer from chronic renal disease.
Diabetes is a major risk factor for renal disease. According to Novo Nordisk, the issue affects around 40% of Type 2 diabetes patients and can lead to further illnesses such as an increased risk of cardiovascular complications and mortality.
“All chronic kidney disease is progressive. It’s a year-on-year, relentless decline in renal function,” Stephen Gough, Novo Nordisk’s global chief medical officer, said in an interview, referring to the kidney’s ability to filter waste from the blood.
Chronic renal disease is a prevalent consequence among persons with type 2 diabetes. Diabetes is the major cause of kidney failure, which is one of the top causes of mortality in the US and globally; according to the US Centers for Disease Control and Prevention, almost one-third of individuals with diabetes also have chronic renal disease.
The clinical research evaluated Ozempic's efficacy when added to conventional care, rather than as a replacement for existing disease-management therapies such as blood pressure medications such as ACE inhibitors and ARBs. The experiment, which began in 2019, included over 3,500 individuals from 28 countries and lasted an average of 3½ years.
The FDA approved the expanded indications on Tuesday, making Ozempic the most extensively indicated medicine in its class, with the widest range of particular medical disorders that it may be prescribed to treat.
The approval also confirms that GLP-1s, a popular family of diabetes and weight reduction medications, offer considerable health advantages beyond than managing blood sugar and decreasing hunger.
Patients who took Ozempic had slower declines in renal function, an 18% lower risk of severe cardiovascular events such as heart attacks, and a 20% lower risk of mortality from any cause compared to those who received placebo. Ozempic also reduced the risk of cardiovascular-related fatalities by 29 percent.
“We know that, unfortunately, cardiovascular disease and chronic kidney disease just go hand in hand,” Gough said.
He went on to say that the main therapies patients receive when they have the first indications of chronic renal disease are designed to lower cardiovascular risk factors by monitoring blood pressure.
“From my point of view as a doctor, you don’t get [diabetes, obesity, chronic kidney disease and cardiovascular disease] in isolation,” Gough said. “These illnesses, unfortunately, co-segregate. They cluster within the same individuals. So if you have a medicine that can target each of these co-morbidities in one injection, then you’re addressing what really matters to the patient.”
Ozempic and other semaglutide injections, such as Wegovy, have been in low supply for years due to their high demand. The FDA now lists Ozempic as “available,” but there is still high demand.
“This is a medicine that’s already available, both in diabetes clinics and now in renal clinics, so I would hope the uptake would start pretty quickly,” Gough said, adding that he hopes the confidence that comes with the data behind this approval will aid clinicians who are making daily decisions about how and when to use different treatments.
“It does help clinicians with their decision-making process, and it helps them focus those medicines to the patients who will benefit the most,” he said.